Palladium-Catalyzed Regiocontrollable Reductive Heck Reaction of Unactivated Aliphatic Alkenes
Chengdong Wang,aGuanlin Xiao,aTao Guo,aYalan Ding,aXiaojin Wu*,aand Teck-Peng Loh*,ab
aInstitute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, Nanjing 211816, P. R. China
bDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637616
Abstract:A general Pd-catalyzed intermolecular reductive Heck reaction of both terminal and internal unactivated aliphatic alkenes has been firstly developed. This method affords γ- and δ-arylated alkyl carboxylic acid derivatives in high yields with complete anti-Markovnikov selectivity. Notably, the coupling process is stereoretentive for the alkyl chain. Mechanistically, alkyl palladacycle intermediates stabilized by directing group and ligand, hydride species multi-generated from PS/TFA reductant, are two key factors that successfully promote the reaction and regioselectivity.
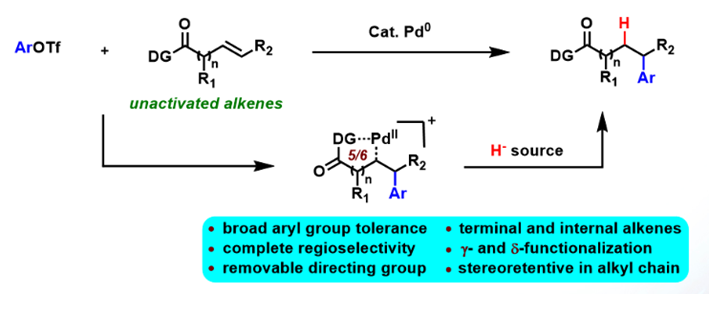
JACS.2018,DOI: 10.1021/jacs.8b03619(2017年影响因子: 13.858,第一作者为硕士研究生王成东).
论文链接:https://pubs.acs.org/doi/pdf/10.1021/jacs.8b03619
(转载自:http://ias.njtech.edu.cn/ch/view.asp?id=1802&class=165)
(审核:杨文忠)
