Selective C(sp3)-H Functionalization of Alkyl Esters withN-/S-/O-Nucleophiles Using Perfluoroalkyl Iodide as Oxidant
Shi-Wen Zhao,aSong-Zhou Cai,aMao-Lin Wang,aWeidong Rao,bHaiyan Xu,cLei Zhang,aXue-Qiang Chu,a,* and Zhi-Liang Shena,*
aInstitute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, People’s Republic of China.
bJiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-Forest Biomass, College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, People’s Republic of China.
cSchool of Environmental and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang, Jiangsu 212003, People’s Republic of China.
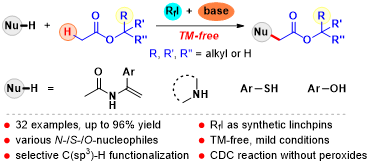
Abstract:An efficient transition metal-free approach to achieve the selective cleavage of the α-carbonyl C(sp3)-H bond in alkyl esters by using inexpensive, low-toxic, andinsensitive perfluoroalkyl iodide as the radical initiator has been developed. A variety of enamides,N-heterocycles, amides,thiophenols, and phenols could be successfully incorporated intofunctionalized alkyl groups by intermolecular amination, thioetherification, and etherification. The distinguishing features of this CDC reaction are its broad substrate scope, synthetic simplicity, and mild reaction conditions.
Adv. Synth. Catal.2020,362, 3388–3394(Impact factor: 5.851).
论文链接:https://onlinelibrary.wiley.com/doi/abs/10.1002/adsc.202000199
