Iron-Catalyzed Tertiary Alkylation of Terminal Alkynes with1,3-Diesters via a Functionalized Alkyl Radical
Ming-Qing Tian,a,+Zhen-Yao Shen,a,+Xuefei Zhao,aPatrick J. Walsh,b,* and Xu-Hong Hua,*
aInstitute of Advanced Synthesis,School of Chemistry and Molecular Engineering,Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816 (China)
bRoy and Diana Vagelos Laboratories, Penn/Merck Laboratory for High-Throughput Experimentation, Department of Chemistry, University of Pennsylvania, 231 South 34th Street, Philadelphia, PA 19104 (USA)
+These authors contributed equally.
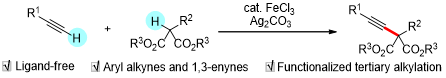
Abstract:Direct oxidative C(sp)-H/C(sp3)-H cross-couplingoffers an ideal and environmentally benign protocol forC(sp)-C(sp3) bond formations. As such, reactivity and site-selectivitywith respect to C(sp3)-H bond cleavage haveremained a persistent challenge. Herein is reported a simple method for iron-catalyzed/silver-mediated tertiary alkylationof terminal alkynes with readily available and versatile 1,3-dicarbonyl compounds. The reaction is suitable for an array of substrates and proceeds in a highly selective manner evenemploying alkanes containing other tertiary, benzylic, and C(sp3)-H bonds alpha to heteroatoms. Elaboration of theproducts enables the synthesis of a series of versatile buildingblocks. Control experiments implicate the in situ generation ofa tertiary carbon-centered radical species.
Angew. Chem. Int. Ed.,Doi: 10.1002/anie.202100641 (2019年影响因子:12.959)
论文链接:https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202100641
