Defluorinative Alkylation of Trifluoromethyl Alkenes with Soft Carbon Nucleophiles Enabled by a Catalytic Amount of Base
Ya Gao, Wei Qin, Ming-Qing Tian, Xuefei Zhao,* and Xu-Hong Hu*
Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, People’s Republic of China
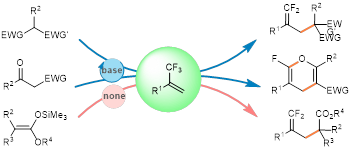
Abstract:Direct manipulation of readily accessibletrifluoromethyl alkenes (TAs) represents an attractiveapproach to the preparation of diversifiedfluorine-containing compounds. In this study, defluorinativealkylation reactions of TAs with a broadarray of soft carbon nucleophiles have beendocumented. Nucleophilic substitutions occur enabledby a catalytic amount of base, providingaccess to tertiary alkyl substituted gem-difluoroalkenesand 2-fluoro-4H-pyrans. By extending thenucleophiles to silyl enol ethers, defluorination canbe achieved in the absence of base to give gemdifluoroalkenes.This process, which eliminates therequirement of organometallic reagents, transitionmetals, or strong bases for the C-F bond cleavage,is applicable to late-stage modification of complexmolecules.
Adv. Synth. Catal.2022,364, 2241-2247(2022年影响因子:5.981)
论文链接:https://onlinelibrary.wiley.com/doi/10.1002/adsc.202200328
被《今日论文》报道评述:https://mp.weixin.qq.com/s/ikM4Usn9oADhL6HtE4Yedw
