Inorganic Chemistry for Tailored Design of Crystalline Nanoporous Materials
Yusuke Yamauchi
University of Wollongong, Squires Way, North Wollongong, NSW 2500, Australia
Abstract: The research on mesoporous (nanoporous) materials, conducted mainly by using surfactant assemblies as templates, has been increasing rapidly. The specific features of regular pore arrangement, uniform mesopore size, and high surface area make these materials very promising for various applications. In this presentation, we will summarize our recent progress on this field.
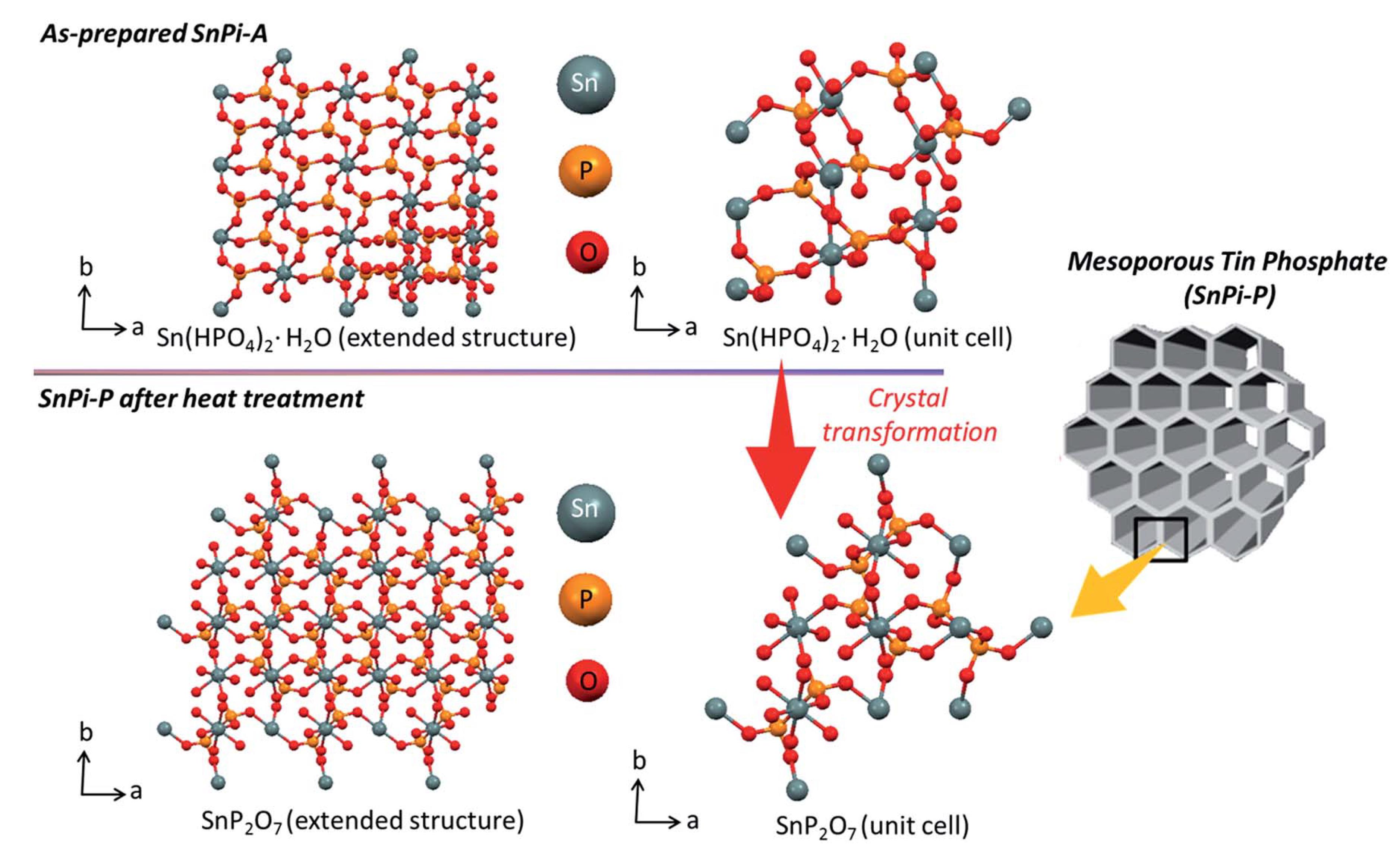
(1) A mesostructured tin phosphate (SnPi) material has been prepared in the presence of an amphiphilic block copolymer (F127), tin chloride (SnCl4), and phosphoric acid (H3PO4) in an ethanol-water mixed solvent medium. The mesoporous SnPi material, with a large pore diameter (7.4 nm), nanocrystalline walls, a high surface area (310 m2 g−1), and a unique flake-like particle shape, is successfully synthesized by a two-step heat treatment of the parent mesostructured SnPi under flowing N2 gas followed by flowing air. Under moderate conditions (293 K and 60% relative humidity), our mesoporous SnPi shows 6.5 times more proton (H+) conductivity compared to its non-porous/bulk analogue (SnPi-B), due to its higher surface area and unique nanocrystalline porous structure enriched with free O–H groups.
(2) We demonstrate a simple and versatile method to grow monodisperse CH3NH3PbBrxIx-3 perovskite nanocrystals inside mesoporous silica templates. The size of the nanocrystal is governed by the pore size of the templates (3.3, 3.7, 4.2, 6.2, and 7.1 nm). Quantum confinement was obse rved by tuning the size of the particles via the template. This approach provides an additional route to tune the optical bandgap of the nanocrystal. Photoluminescence measurements on CH3NH3PbBr clearly show a shift from green to blue as the pore size is decreased.
Selected Publications in 2015-2016:Nature Chemistry, 8, 638 (2016);Angew. Chem. Int. Ed., 55, 10037 (2016);Angew. Chem. Int. Ed., 55, 8426 (2016);Angew. Chem. Int. Ed., 55, 8228 (2016);Angew. Chem. Int. Ed., 55, 12746 (2016);Angew. Chem. Int. Ed., 55, 12793 (2016);J. Am. Chem. Soc., 138, 13874 (2016);Nature Communications, 6, Article number: 6608 (2015);Angew. Chem. Int. Ed., 54, 11073 (2015);Angew. Chem. Int. Ed., 54, 4222 (2015);Angew. Chem. Int. Ed., 54, 951 (2015);Angew. Chem. Int. Ed., 54, 588 (2015);J. Am. Chem. Soc., 137, 11558 (2015);J. Am. Chem. Soc.,137, 1572 (2015).
