报告时间:2016年11月2日下午14:30
报告地点:科技创新大楼C501室
报告题目:1D-, 2D-, AND 3D-COORDINATION POLYMERS AND METAL-ORGANIC FRAMEWORKS CONTAINING MAIN GROUP ELEMENTS

Professor Paul D. Lickiss
Department of Chemistry, Imperial College London, SW7 2AZ UK
Paul Lickiss obtained both his BSc. (1980) and DPhil. (1983) from the University of Sussex, where his DPhil. was supervised by Professor C. Eaborn, FRS. He left Sussex to work as a postdoctoral fellow with Professor A. G. Brook in Toronto where he prepared some of the first compounds to contain silicon to carbon double bonds. He returned to Sussex and was awarded one of the newly set up Royal Society 1983 University Research Fellowships. He resigned this Fellowship in 1989 to take up a lectureship at the University of Salford. After four years at Salford Professor Lickiss moved to London to take up a lectureship at Imperial College where he was promoted to Senior Lecturer in 1999, to Reader in Organometallic Chemistry in 2001 and to Professor of Organometallic Chemistry in 2015.
The Lickiss research group has a range of interests in the field of main-group chemistry, particularly organosilicon chemistry. The general areas of interest have been the chemistry of bulky organosilicon compounds and reactive intermediates derived from them such as silyl cations. Silanols, siloxanes and silsesquioxanes have also been continuing areas of interest. More recently, the chemistry of metal-organic frameworks as materials for hydrogen storage, carbon capture and drug delivery has been a focus in the group. This work has concentrated on the use of main group elements such as magnesium as nodes in the frameworks, and on organosilicon linkers.
1D-, 2D-, AND 3D-COORDINATION POLYMERS AND METAL-ORGANIC FRAMEWORKS CONTAINING MAIN GROUP ELEMENTS
Paul D. Lickiss
Chemistry Department, Imperial College London, South Kensington, London SW7 2AZ, UK
e-mail:p.lickiss@imperial.ac.uk
In contrast to the very large volume of studies on metal-organic frameworks (MOFs) and other coordination polymers based on transition metals, relatively few studies have been carried out with either the nodes or the linkers being main group elements. This lecture will consider firstly some comparisons of well known Zn MOFs with analogous compounds containing Mg nodes. Secondly, and more importantly the potential for organosilicon linkers to be used in MOFs will be described. Thus, disiloxanes containing pyridyl groups can form coordination polymers with a range of metals such as Mn(II), Co(II), and Cu(II) [1]. The utility of such ligands for coordination polymer formation is based on their flexibility in the case of the disiloxanes, and upon the convenient synthesis of new ligands containing several organic ligating groups via simple synthetic steps at tetrahedral Si-centres to give compounds of the type RnSi(p-C6H4CO2H)4-n(n = 0, 1, or 2; R = Me, Et, Ph etc.).
Treatment of transition metal salts and main group element precursors with Si(p-C6H4CO2H)4affords a variety of MOFs that contain either the [Si(p-C6H4CO2)4]4-or [Si(p-C6H4CO2)3(p-C6H4CO2H)]3-anion as the linker [2, 3]. More complicated arylsilanes such as C6H4-p-[(SiC6H4CO2H)3]2and [CH2(SiC6H4CO2H)3]2also give rise to the formation of novel framework materials when treated with metal salts. For example, the figure below shows one pore in the product derived from reaction of zinc nitrate with the hexafunctional organosilicon linker C6H4-p-[(SiC6H4CO2H)3]2.The structure is a MOF isoreticular to the well-known MOF5, but with alternate corners occupied by Si rather than by Zn4O6+clusters (indicated by the tetrahedra) [4]. The potential of these carboxylate linkers as well as other tetrazolate linkers in providing a wide range of MOF structures will be described.
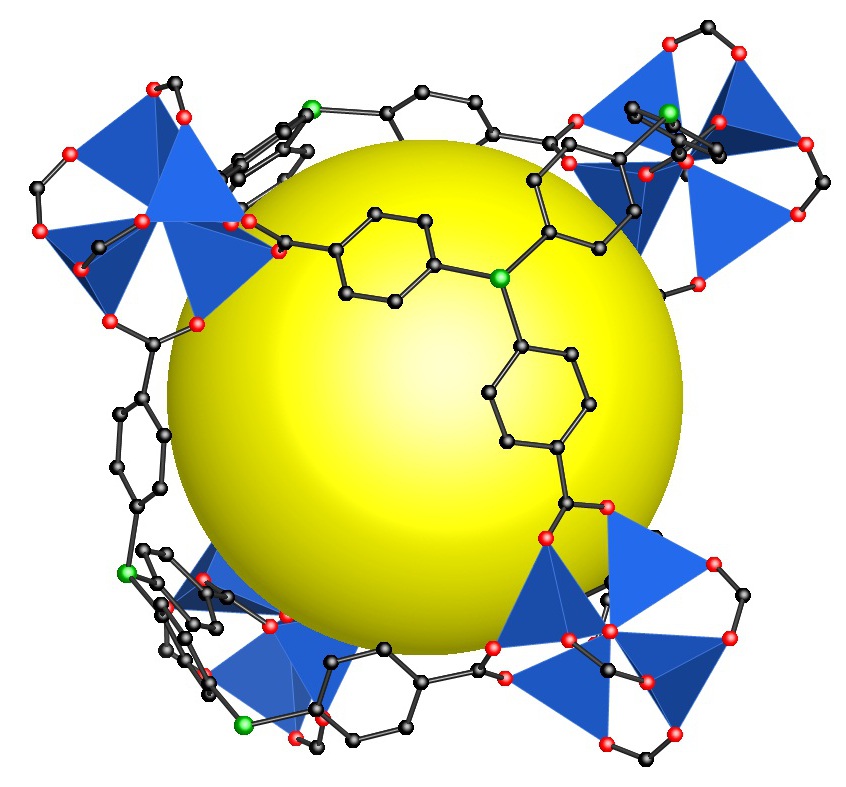
________________________________
1. D. M. L. Goodgame, P. D. Lickiss, S. J. Rooke, A. J. P. White and D. J. Williams, Inorg. Chim. Acta, 2003 343, 61;
2. R. P. Davies, R. J. Less, P.D. Lickiss, K. Robertson, A. J. P. White, Inorg, Chem., 2008, 47, 9958;
3. R. P. Davies, R. Less, P. D. Lickiss, K. Robertson and A. J. P. White, Crystal Growth and Design, 2010, 10, 4571;
4 R. P. Davies, P. D. Lickiss, K. Robertson, A. J. P. White, Crystengcomm, 2012, 14, 758.
